Zacks
Anavex’s (AVXL) Lead Alzheimer’s Drug Meets Study Goal
Zacks Equity Research – December 5, 2022
Shares of Anavex Life Sciences AVXL were up 35.9% on Dec 2 after management reported positive topline data from a phase IIb/III study which evaluated its lead pipeline candidate ANAVEX 2-73 (blarcamesine) in Alzheimer’s disease (AD) indication.
The phase IIb/III study, or the ANANVEX 2-73-AD-004 study, evaluated ANAVEX 2-73 for the treatment of mild cognitive impairment (MCI) due to AD and mild AD (collectively known as early AD)
The ANAVEX 2-73-AD-004 study achieved its primary and key secondary endpoints. Treatment with ANAVEX 2-73 showed robust, statistically significant and clinically meaningful absolute improvement in cognitive functions as measured by ADAS-Cog and ADCS-ADL that were the study’s primary endpoints over a 48-week treatment period in the analysis of the intent-to-treat (ITT) population.
Data from the study showed that study participants who received ANAVEX 2-73 were 84% more likely to have improved cognition than those who were administered placebo. Patients treated with ANAVEX 2-73 were 167% more likely to improve function than those participants who were receiving a placebo. The treatment also showed a statistically significant reduction in cognitive decline at the end of treatment by 45%, when compared with placebo.
The study also met its secondary endpoint of reduction in clinical decline of cognition and function, as measured by CDR-SB score. Data from the study showed a 27% reduction in the ITT population when compared to placebo-administered participants.
The ANAVEX 2-73-AD-004 study randomized AD participants into three equal groups – one group which received a mid-dose of ANAVEX 2-73, a second group, which received a high-dose of the drug and a third group which received placebo.
Anavex continues to conduct a further analysis the above data and intends to submit the same for publication in a peer-reviewed medical journal. Management is also conducting an open-label extension study ATTENTION-AD to follow study participants over a 96-week treatment period.
Shares of Anavex have declined 30.5% this year compared with the industry’s 16.7% fall.
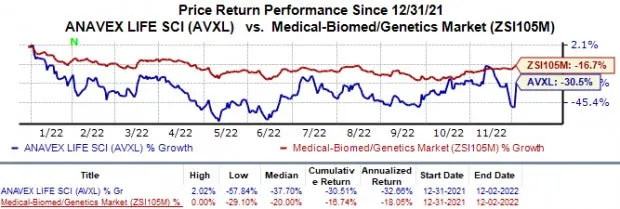
Image Source: Zacks Investment Research
The results of the ANAVEX 2-73-AD-004 study are also consistent with the phase IIa ANAVEX 2-73 study previously conducted by the company. Data from the phase IIa study had demonstrated a therapeutic effect on cognition and function.
Per management, AD is one of the leading causes of deaths in older adults aged above 65 years and is also the most common cause of dementia in this age group. Treatment with ANAVEX 2-73 demonstrated a reversal of cognitive decline.
Apart from AD, Anavex has also successfully completed clinical studies evaluating ANAVEX 2-73 in other indications. These include a phase II proof-of-concept study on Parkinson’s disease dementia and a phase III study in adult patients with Rett syndrome.
Anaex’s target market is highly competitive as several other pharma companies like Biogen BIIB and Eli Lilly LLY are also developing their candidates targeting the AD indication. The Alzheimer’s candidates of these companies — anti-amyloid beta antibodies — are in late-stage development or review and are expected to be launched in a few months.
Last week, Biogen along with partner Eisai presented detailed data from the phase III confirmatory study CLARITY AD, which evaluated its AD candidate lecanemab (BAN2401) to treat early AD. The data showed that Biogen’s candidate did reduce markers of amyloid in early Alzheimer’s disease and led to moderately less decline in measures of cognition and function than placebo at 18 months. However, treatment with lecanemab was associated with adverse events.
Biogen/Eisai have already filed their biologics license application (BLA) seeking accelerated approval for lecanemab with the FDA, supported by data from a phase II study (Study 201). A final BLA decision is expected by Jan 6, 2023.
Eli Lilly has developed donanemab, an investigational antibody therapy, for AD. Eli Lilly initiated a rolling submission with the FDA last year, seeking approval for donanemab under the accelerated pathway based on data from the phase II TRAILBLAZER-ALZ study. A final decision on the BLA is expected in early 2023. Eli Lilly also expects a data readout from the pivotal phase III TRAILBLAZER-ALZ 2 by mid-2023. If positive, the data will form the basis of its application for traditional regulatory approval for donanemab.
Last month, Roche RHHBY announced the failure of the GRADUATE I and II studies, evaluating its monoclonal antibody gantenerumab in early AD. The studies failed to meet their primary endpoint of slowing clinical decline. Patients treated with Roche’s gantenerumab showed a slowdown of clinical decline in GRADUATE I and GRADUATE II, which was not statistically significant. Per Roche, the level of beta-amyloid removal was lower than expected.
Anavex Life Sciences Corp. Price

Anavex Life Sciences Corp. price | Anavex Life Sciences Corp. Quote
